“The entry of a short administrative stay is standard operating procedure whenever the Supreme Court is asked to consider an emergency application like this. Gives the court enough time to consider the parties’ arguments before ruling. We hope to explain why the FDA has failed to meet its heavy burden of halting the parts of the district court’s decision that restore critical safeguards for women and girls that were unlawfully removed by the FDA.” , he said.
This Supreme Court decision means that, for now, mifepristone remains legal up to 10 weeks pregnant, and can be mailed and administered via telemedicine without an in-person doctor’s visit.
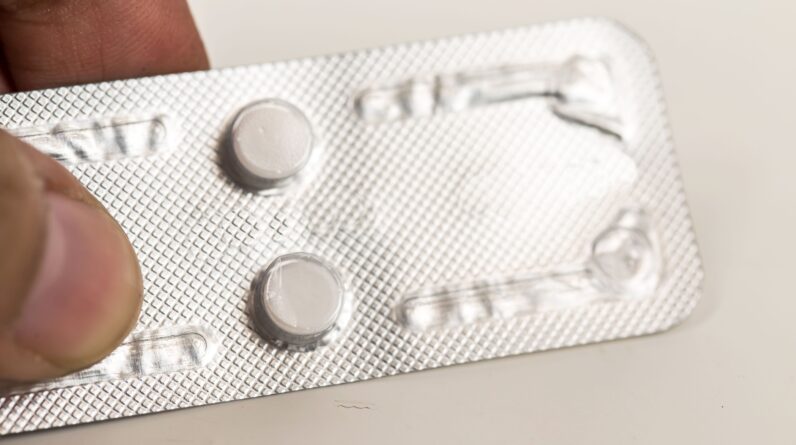
Mifepristone is the first drug used in what is usually a two-step regimen for a chemical abortion. The pill works to kill an unborn baby by cutting off the nutrients it needs to continue developing.
Chemical abortions now account for more than half of all abortions in the United States.
A Texas judge’s decision last Friday to suspend FDA approval of the abortion drug mifepristone was made on the grounds that the approval was “based on clearly flimsy reasoning and studies that did not support its conclusions.” .
On Thursday, a three-judge panel of the Louisiana 5th Circuit Court of Appeals granted a partial stay of Kacsmaryk’s April 7 sentence while reinstating certain restrictions on mifepristone lifted by the FDA after 2016.
[ad_2]
Source link





